Page 189 - CW E-Magazine (3-12-2024)
P. 189
Special Report
system. The enthalpy changes (kcal
mol ) associated with some relevant
-1
reactions is noted below.
Cl (g) + H O(l) HOCl(aq) + HCl(aq)
2
2
Δ H = +0.29
Fe + HOCl(aq) + HCl (aq) FeCl (aq)
2
+ H O(l) ΔH = -100.29
2
Fe + 2HCl(aq) FeCl (aq) + H (g)
2
2
ΔH = -20.30
H (g) + Cl (g) 2HCl(g)
2
2
ΔH = -44.12
R-H(l) + Cl (g) R-Cl(l) + HCl(g)
2
Δ H = -30.00
Simple heat balance calculations
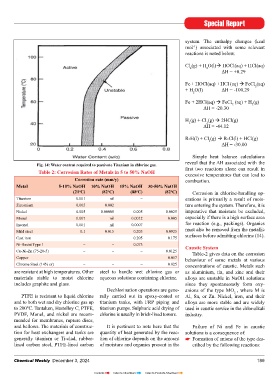
Fig. 14: Water content required to passivate Titanium in chlorine gas. reveal that the ΔH associated with the
Table 2: Corrosion Rates of Metals in 5 to 50% NaOH first two reactions alone can result in
excessive temperatures that can lead to
Corrosion rate (mm/y) combustion.
Metal 5-10% NaOH 10% NaOH 15% NaOH 30-50% NaOH
(21 C) (82 C) (88 C) (82 C) Corrosion in chlorine-handling op-
o
o
o
o
Titanium 0.001 nil – erations is primarily a result of mois-
Zirconium 0.005 0.002 – ture entering the system. Therefore, it is
Nickel 0.005 0.00008 0.005 0.0025 imperative that moisture be excluded,
Monel 0.007 nil 0.0012 0.005 especially if there is a high surface area
Inconel 0.001 nil 0.0007 for reaction (e.g., packings). Organics
Mild steel 0.1 0.015 0.205 0.0925 must also be removed from the metallic
Cast iron – – 0.205 0.175 surfaces before admitting chlorine (14).
Ni-Resist Type 1 – – 0.073 Caustic System
Cu-Ni-Zn (75-20-5) – – – 0.0125 Table-2 gives data on the corrosion
Copper – – – 0.057 behaviour of some metals at various
Chrome Steel (14% cr) – – – 0.825 concentrations of caustic. Metals such
are resistant at high temperatures. Other steel to handle wet chlorine gas or as aluminium, tin, and zinc and their
materials stable to moist chlorine aqueous solutions containing chlorine. alloys are unstable in NaOH solutions
includes graphite and glass. since they spontaneously form oxy-
Dechlorination operations are gene- anions of the type MO , where M is
–
2
PTFE is resistant to liquid chlorine rally carried out in epoxy-coated or Al, Sn, or Zn. Nickel, iron, and their
and to both wet and dry chlorine gas up titanium tanks, with FRP piping and alloys are more stable and are widely
to 200°C. Tantalum, Hastelloy C, PTFE, titanium pumps. Sulphuric acid drying of used in caustic service in the chlor-alkali
PVDF, Monel, and nickel are recom- chlorine is usually in brick-lined towers. industry.
mended for membranes, rupture discs,
and bellows. The materials of construc- It is pertinent to note here that the Failure of Ni and Fe in caustic
tion for heat exchangers and tanks are quantity of heat generated by the reac- solutions is a consequence of:
generally titanium or Ti-clad, rubber- tion of chlorine depends on the amount Formation of anions of the type des-
lined carbon steel, PTFE-lined carbon of moisture and organics present in the cribed by the following reactions:
Chemical Weekly December 3, 2024 189
Contents Index to Advertisers Index to Products Advertised