Page 185 - CW E-Magazine (3-12-2024)
P. 185
Special Report
a pH of 14 due to the following
reactions; and:
Fe + 2H Fe + + H (pH < 8)
2
2
+
Fe + 2H O Fe + + 2OH + H (pH
–
2
2
2
9-10)
Fe + OH + H O HFeO - + H (pH
–
2
2
2
> 14)
Reaction of iron with hypochlorite:
Fe + OCl- +H O Fe + + 20H + Cl –
+
–
2
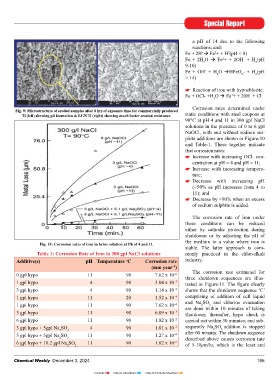
Corrosion rates determined under
Fig. 9: Microstructure of eroded samples after 8 hrs of exposure time for commercially produced
Ti (left) showing pit formation & LGN Ti (right) showing much better erosion resistance. static conditions with steel coupons at
90°C at pH 4 and 11 in 300 gpl NaCl
solutions in the presence of 0 to 6 gpl
NaOCl, with and without sodium sul-
phite additions are shown in Figure-10
and Table-1. These together indicate
that corrosion rates:
Increase with increasing OCl- con-
centration at pH = 4 and pH = 11;
Increase with increasing tempera-
ture;
Decrease with increasing pH
(~50% as pH increases from 4 to
11); and
Decrease by >90% when an excess
of sodium sulphite is added.
The corrosion rate of iron under
these conditions can be reduced
either by cathodic protection during
shutdowns or by adjusting the pH of
the medium to a value where iron is
Fig. 10: Corrosion rates of iron in brine solution at Ph of 4 and 11.
stable. The latter approach is com-
Table 1: Corrosion Rate of Iron in 300 gpl NaCI solutions monly practiced in the chlor-alkali
Additive(s) pH Temperature C Corrosion rate industry.
o
(mm year )
–1
The corrosion rate estimated for
0 gpl hypo 11 90 7.62 x 10 –6 three shutdown sequences are illus-
1 gpl hypo 4 90 3.04 x 10 –3 trated in Figure-11. The figure clearly
3 gpl hypo 4 90 1.14 x 10 –2 shows that the shutdown sequence ‘C’
1 gpl hypo 11 20 1.52 x 10 –4 comprising of addition of cell liquid
1 gpl hypo 11 90 7.62 x 10 –4 and Na SO and chlorine evacuation
3
2
are done within 10 minutes of taking
3 gpl hypo 11 90 6.09 x 10 –3 shutdown; thereafter, hypo check is
6 gpl hypo 11 90 1.82 x 10 –2 carried out within 30 minutes; and sub-
3 gpl hypo + 5gpl Na SO 4 90 1.01 x 10 –3 sequently Na SO addition is stopped
2 3 2 3
3 gpl hypo + 5gpl Na SO 11 90 1.27 x 10 –4 after 60 minutes. The shutdown sequence
2 3 described above causes corrosion rate
6 gpl hypo + 10.2 gpl Na SO 3 11 90 1.02 x 10 –5 of 5-10μm/hr, which is the least and
2
Chemical Weekly December 3, 2024 185
Contents Index to Advertisers Index to Products Advertised