Page 190 - CW E-Magazine (3-12-2024)
P. 190
Special Report
–
Ni + H O + OH- HNiO + H (9) solve in highly concentrated NaOH- hydrogen can diffuse into the metal, caus-
2
2
2
–
Fe + H O + OH- HFeO + H (10) solutions, and the corrosion domains ing hydrogen embrittlement and SCC.
2
2
2
become larger as the temperature in-
Oxidation of Fe and Ni following creases. The active dissolution of iron Carbon steel
the reduction of OCl or ClO ions. or nickel in NaOH is accompanied by Carbon steel is commonly used
–
–
3
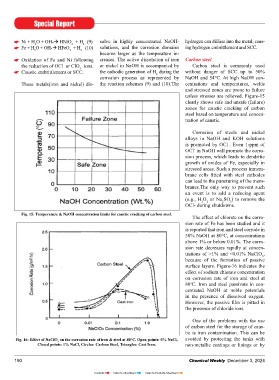
Caustic embrittlement or SCC. the cathodic generation of H during the without danger of SCC up to 50%
2
corrosion process as represented by NaOH and 54°C. At high NaOH con-
These metals(iron and nickel) dis- the reaction schemes (9) and (10).The centrations and temperatures, welds
and stressed zones are prone to failure
unless stresses are relieved. Figure-15
clearly shows safe and unsafe (failure)
zones for caustic cracking of carbon
steel based on temperature and concen-
tration of caustic.
Corrosion of steels and nickel
alloys in NaOH and KOH solutions
is promoted by OCl . Even 1-ppm of
–
OCl in NaOH will promote the corro-
–
sion process, which leads to dendritic
growth of oxides of Fe, especially in
stressed areas. Such a process inmem-
brane cells fitted with steel cathodes
can lead to the puncturing of the mem-
branes.The only way to prevent such
an event is to add a reducing agent
(e.g., H O or Na SO ) to remove the
2
2
3
2
OCl- during shutdowns.
Fig. 15: Temperature & NaOH concentration limits for caustic cracking of carbon steel.
The effect of chlorate on the corro-
sion rate of Fe has been studied and it
is reported that iron and steel corrode in
50% NaOH at 80°C, at concentrations
above 1% or below 0.01%. The corro-
sion rate decreases rapidly at concen-
trations of >1% and <0.01% NaClO ,
3
because of the formation of passive
surface layers. Figure-16 indicates the
effect of sodium chlorate concentration
on corrosion rate of iron and steel at
80 C. Iron and steel passivate in con-
o
centrated NaOH at noble potentials
in the presence of dissolved oxygen.
However, the passive film is pitted in
the presence of chloride ions.
One of the problems with the use
of carbon steel for the storage of caus-
tic is iron contamination. This can be
Fig. 16: Effect of NaClO on the corrosion rate of iron & steel at 80°C. Open points: 0% NaCl, avoided by protecting the tanks with
3
Closed points: 1% NaCl, Circles: Carbon Steel, Triangles: Cast Iron. non-metallic coatings or linings or by
190 Chemical Weekly December 3, 2024
Contents Index to Advertisers Index to Products Advertised