Page 193 - CW E-Magazine (3-12-2024)
P. 193
Special Report
its alloys are used for vapour bodies, a factor of 10-20, depending on the releasing oxygen according to the
piping, and heat exchangers in evapo- nature of the weld and the methods following reaction.
rator systems and for operations where employed to relieve stress.
high solution velocities are encountered 2NaClO 2NaCl + 3O (11)
3
2
(e.g., centrifugal pumps). The presence of hypochlorite in
caustic is deleterious to nickel evapora- The oxidation of nickel follows the
Thermodynamically, nickel should tors, forcing the oxidation of nickel to following reactions:
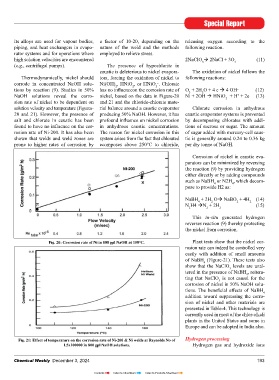
corrode in concentrated NaOH solu- Ni(OH) , HNiO , or HNiO . Chlorate
–
2
2
2
tions by reaction (9). Studies in 50% has no influenceon the corrosion rate of O + 2H O + 4 e 4 OH (12)
–
2
2
NaOH solutions reveal the corro- nickel, based on the data in Figure-20 Ni + 2OH HNiO + H + 2e (13)
–
–
+
2
sion rate of nickel to be dependent on and 21 and the chloride-chlorate mate-
solution velocity and temperature (Figures- rial balance around a caustic evaporator Chlorate corrosion in anhydrous
20 and 21). However, the presence of producing 50% NaOH. However, it has caustic evaporator systems is prevented
salt and chlorate in caustic has been profound influence on nickel corrosion by decomposing chlorates with addi-
found to have no influence on the cor- in anhydrous caustic concentrations. tions of sucrose or sugar. The amount
rosion rate of Ni-200. It has also been The reason for nickel corrosion in this of sugar added with mercury-cell caus-
shown that welds and weld zones are system arises from the fact that chlorated tic is generally around 0.24 to 0.36 kg
prone to higher rates of corrosion by ecomposes above 250°C to chloride, per dry tonne of NaOH.
Corrosion of nickel in caustic eva-
porators can be minimized by reversing
the reaction (9) by providing hydrogen
either directly or by adding compounds
such as NaBH or N2H , which decom-
4
4
pose to provide H2 as:
NaBH + 2H O NaBO + 4H (14)
2
2
2
4
N H4 N + 2H (15)
2
2
2
This in-situ generated hydrogen
reverses reaction (9) thereby protecting
the nickel from corrosion.
Fig. 20: Corrosion rate of Ni in 800 gpl NaOH at 158°C. Plant tests show that the nickel cor-
rosion rate can indeed be controlled very
easily with addition of small amounts
of NaBH (Figure-21). These tests also
4
show that the NaClO levels are unal-
3
tered in the presence of NaBH , reitera-
4
ting that NaClO is not causal for the
3
corrosion of nickel in 50% NaOH solu-
tions. The beneficial effects of NaBH
4
addition toward suppressing the corro-
sion of nickel and other materials are
presented in Table-4. This technology is
currently used in most of the chlor-alkali
plants in the United States and some in
Europe and can be adopted in India also.
Fig. 21: Effect of temperature on the corrosion rate of Ni-200 & Ni welds at Reynolds No of Hydrogen processing
1.5x100000 in 800 gpl NaOH solutions. Hydrogen gas and hydroxide ions
Chemical Weekly December 3, 2024 193
Contents Index to Advertisers Index to Products Advertised