Page 194 - CW E-Magazine (3-12-2024)
P. 194
Special Report
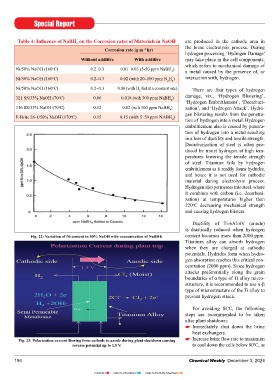
Table 4: Influence of NaBH on the Corrosion rates of Materials in NaOH are produced in the cathode area in
4
Corrosion rate (g m hr) the brine electrolysis process. During
–2
hydrogen processing ‘Hydrogen Damage’
Without additive With additive may take place in the cell components,
which refers to mechanical damage of
Ni/50% NaOH (160 C) 0.2–0.3 0.01–0.03 (5-50 ppm NaBH )
o
4 a metal caused by the presence of, or
Ni/50% NaOH (160 C) 0.2–0.3 0.02 (with 20–100 ppm N H ) interaction with, hydrogen.
o
2 4
Ni/50% NaOH (160 C) 0.2–0.3 0.08 (with H fed at a constant rate)
o
2 There are four types of hydrogen
321 SS/33% NaOH (70 C) 0.06 0.018 (wih 300 ppm NaBH ) damage, viz., ‘Hydrogen Blistering’,
o
4
‘Hydrogen Embrittlement’, ‘Decarburi-
316 SS/33% NaOH (70 C) 0.12 0.02 (wih 300 ppm NaBH ) zation’, and ‘Hydrogen Attack’. Hydro-
o
4
gen blistering results from the penetra-
E-Brite 26-1/50% NaOH (170 C) 0.15 0.15 (with 5–50 ppm NABH )
o
4 tion of hydrogen into a metal. Hydrogen
embrittlement also is caused by penetra-
tion of hydrogen into a metal resulting
in a loss of ductility and tensile strength.
Decarburization of steel is often pro-
duced by moist hydrogen at high tem-
peratures lowering the tensile strength
of steel. Titanium fails by hydrogen
embrittlement as it readily forms hydrides,
and hence it is not used for cathodic
material during electrolysis process.
Hydrogen also permeates into steel, where
it combines with carbon (i.e. decarboni-
zation) at temperatures higher than
220 C decreasing mechanical strength
o
and causing hydrogen blisters.
Ductility of Ti-6Al-4V (anode)
is drastically reduced when hydrogen
Fig. 22: Variation of Ni content in 50% NaOH with concentration of NaBH4. content becomes more than 2000 ppm.
Titanium alloy can absorb hydrogen
when they are charged at cathodic
potentials. Hydrides form when hydro-
gen absorption reaches this critical con-
centration (2000 ppm). Since hydrogen
attacks preferentially along the grain
boundaries of α type of Ti alloy micro-
structure, it is recommended to use α-β
type of microstructure of the Ti alloy to
prevent hydrogen attack.
For avoiding SCC, the following
steps are recommended to be taken
after plant shutdown:
Immediately shut down the brine
heat exchangers.
Fig. 23: Polarization current flowing from cathode to anode during plant shutdown causing Increase brine flow rate to maximum
reverse potential up to 1.5 V. to cool down the cells below 80ºC, as
194 Chemical Weekly December 3, 2024
Contents Index to Advertisers Index to Products Advertised