Page 191 - CW E-Magazine (26-9-2023)
P. 191
Special Report
modynamic efficiency, in the range
of 40-60%, significantly higher than
combustion-based systems. This higher
energy conversion efficiency leads
to improved overall performance and
reduced fuel consumption. The only
by-product from the reaction that
occurs within fuel cells is water. Additio-
nally, fuel cells provide versatility in
terms of fuel options, utilising various
fuels, including hydrogen, natural gas,
methanol, and ethanol. When utilising
fuels other than hydrogen, the quantity
of pollutants emitted is two orders of
magnitude lower than that produced
by conventional sources of electricity.
This brings up the possibility of using
existing infrastructure to provide fuel
cells with their fuels. In terms of power
output, fuel cells are flexible and can
be scaled for power production in the
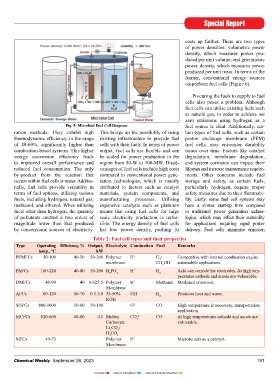
Fig. 5: Microbial Fuel cell Diagram region from 50-W to 100-MW.
dependent on the ion mobility and the can be scaled up for commercial use
electrolyte conductivity. This is why also determine whether a technology’s Disadvantages of fuel cells include
certain fuel cells are better than others, performance is fit for use as an energy high costs compared to conventional
as some may use ions which have source. power generation technologies, which
a higher ion mobility and therefore is mainly attributed to factors such as
offer less resistance. A fuel cell’s perfor- summary catalyst materials, system components,
mance can be evaluated based on its Generally, fuel cells offer several and manufacturing processes. Utilising
efficiency and total output. Other fac- advantages over traditional power gene- expensive catalysts such as platinum
tors such as temperature, or whether it ration methods. They exhibit high ther- means that using fuel cells for large
table 2: Fuel cell types and their properties
type Operating Efficiency, % Output, Electrolyte conduction Fuel remarks
temp., °c kW
PEMFCs 50-100 40-50 50-200 Polymer H + H / Competitive with internal combustion engine –
2
membrane CH OH automobile applications.
3
PAFCs 160-220 40-80 50-200 H PO H + H Acid can corrode the electrodes. At high tem-
3 4 2
peratures cathode and anode are vulnerable.
DMFCs 40-90 40 0.025-5 Polymer H + Methanol Methanol crossover.
Membrane
AFCs 60-120 60-70 0.3-5.0 35-50% OH – H 2 Produces heat and water.
KOH
SOFCs 800-1000 50-60 50-100 O 2– CO High temperature is necessary, transportation
application.
MCFCs 620-660 60-80 0.1 Molten CO 3 2– CO At high temperatures cathode and anode are
Carbonate vulnerable.
Li CO /
2
3
H CO
2 3
MFCs 49-53 Polymer H + Microbe acts as a catalyst.
Membrane
Chemical Weekly September 26, 2023 191
Contents Index to Advertisers Index to Products Advertised