Page 188 - CW E-Magazine (26-9-2023)
P. 188
Special Report
the chemical species. In the combus-
2e - 2e -
tion reaction above, the energy from
V the reconfiguration of electrons can
only be recovered as heat due to the
timescale in which the reaction takes
2e - 2e - place. However, dragging the reaction
across a longer period of time can allow
one to directly utilise the energy of the
charge from the redox reaction. This is
Salt Bridge
achieved by separating the reaction’s
oxidation and reduction components.
In the diagram below, the hydrogen
combustion reaction is split into two
half-reactions, occurring at the anode
Zinc electrode
Copper electrode
and cathode. Hydrogen is bubbled to
the anode, where it loses its electrons to
Zn(s) Zn (aq) + 2e - Cu (aq) Cu(s) the electrode. The electrolyte in which
2e
2+
2+
-
the electrodes are immersed allows for
the free flow of ions while preventing
the electrons from flowing. This forces
the electron to go through the conduc-
Oxidation half cell Reduction half cell tor while the hydrogen ion flows from
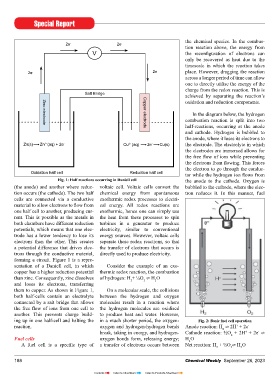
Fig. 1: Half reactions occurring in Daniell cell the anode to the cathode. Oxygen is
(the anode) and another where reduc- voltaic cell. Voltaic cells convert the bubbled to the cathode, where the elec-
tion occurs (the cathode). The two half chemical energy from spontaneous tron reduces it. In this manner, fuel
cells are connected via a conductive exothermic redox processes to electri-
material to allow electrons to flow from cal energy. All redox reactions are
one half cell to another, producing cur- exothermic, hence one can simply use
rent. This is possible as the metals in the heat from these processes to spin
both chambers have different reduction turbines in a generator to produce
potentials, which means that one elec- electricity, similar to conventional
trode has a lower tendency to lose its energy sources. However, voltaic cells
electrons than the other. This creates separate these redox reactions, so that
a potential difference that drives elec- the transfer of electrons that occurs is
trons through the conductive material, directly used to produce electricity.
forming a circuit. Figure 1 is a repre-
sentation of a Daniell cell, in which Consider the example of an exo-
copper has a higher reduction potential thermic redox reaction, the combustion
than zinc. Consequently, zinc dissolves of hydrogen: H + ½O ⇌ H O
2
2
2
and loses its electrons, transferring
them to copper. As shown in Figure 1, On a molecular scale, the collisions
both half-cells contain an electrolyte between the hydrogen and oxygen
connected by a salt bridge that allows molecules result in a reaction where
the free flow of ions from one cell to the hydrogen molecules are oxidised
another. This prevents charge build- to produce heat and water. However,
ing up in one half-cell and halting the in a much shorter period, the oxygen- Fig. 2: basic fuel cell operation
reaction. oxygen and hydrogen-hydrogen bonds Anode reaction: H ⇌ 2H + 2e -
+
2
break, taking in energy, and hydrogen- Cathode reaction: ½O + 2H + 2e ⇌
+
-
2
Fuel cells oxygen bonds form, releasing energy; H O
2
A fuel cell is a specific type of a transfer of electrons occurs between Net reaction: H + ½O ⇌ H O
2
2
2
188 Chemical Weekly September 26, 2023
Contents Index to Advertisers Index to Products Advertised