Page 186 - CW E-Magazine (17-12-2024)
P. 186
Special Report Special Report
has actually helped in the crystallisa- steps was 72%, improving the total route of operability provided by continuous this approach has the following
tion of pure product directly from the yield to 53% from 27% in batch mode. method development that would have advantages over batch process: (i) the
reaction mixture. (Dennehy et al. Org. This work clearly highlights the benefi ts been otherwise unattainable in batch turbo Grignard reagent formation and
Process Res. Dev. 2020, 24, 10, 1978 – of combining batch and fl ow processes mode. subsequent reactions are more effi cient,
1987). for safety, effi ciency, and sustainability improving overall synthesis effi ciency
in pharmaceutical synthesis. Advantages Tramadol compared to conventional methods
Advantages over batch process over batch process include: (i) improved Tramadol is an analgesic drug used with lower yields (< 35%); (ii) only
include: (i) A greener approach by using the total route yield to 53% from 27%; for management of moderate to severe small amounts of reactive or hazardous
reduced solvent volume and a greener and (ii) a total residence time for the four pain. It was synthesized with improved intermediates (like Grignard reagents)
solvent; (ii) method enables inline purifi - steps is 90 min. yield (96%) in a continuous fl ow reac- are present at any time; and (iii)
cation and separation, resulting in the tor, in comparison to traditional batch importantly, reduced reaction times for
crystallisation of pure product directly Grignard reactions process. A multi-operation, continuous- Grignard formation was reported.
from the reaction mixture; and (iii) Synthesis of Grignard reagent and fl ow platform for the synthesis of tra-
complete removal of 2.1 equivalents of its subsequent reaction are pivotal to madol, ranging from gram to decagram Nitration and Diazotization
the succinimide by-product. not only pharma industry but also the quantities, is described by Monos et These are among the most commonly
agrochemical industry. Few reports on al. (Synlett 2020; 31(19): 1888-1893). done reactions in pharma intermediate
Cinnarizine, cyclizine, buclizine, synthesis of some important drugs are A comparison of process metrics synthesis. Incidentally, in most of the
and meclizine belong to a family of described below- including E-Factor, production rate, cases these are done in batch mode at
antihistamines that resemble each other and space-time yield are used to con- conditions of low temperature, slow
in terms of a 1-diphenylmethylpiperazine Fluconazole textualize the developed platform with addition of one of the reagents, use of
moiety. A four-step continuous process Fluconazole is a triazole compound, respect to established engineering and large quantities of solvents as well as
subsequent continuous removal and intermediate isolation. (De Vitis et al., to generate the fi nal antihistamines widely used anti-fungal, also found use- synthetic methods for making tramadol. reagents, etc. In the recent time, it has
mitigation of HCl that evolves needs ChemistryOpen. 2017, 6(5):668-673.) from bulk alcohols has been reported by ful in HIV-infected and cancer patients. been demonstrated for a large number
special attention. Borukhova et al. (ChemSusChem 2016, The drug was commercialized way Advantages of this approach over of substrates that these reactions are
Advantages over batch process 9, 67-74). HCl is used to synthesize the back in 1988 with a tradename, Difl ucan. the batch process include: (i) all purifi - better when done in fl ow.
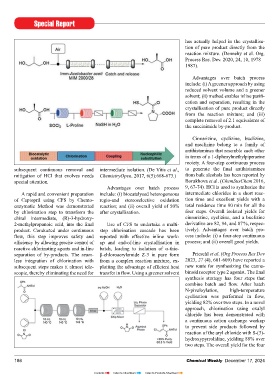
A rapid and convenient preparation include: (i) biocatalysed heterogeneous intermediate chlorides in a short reac- Korwar et al. (Eur. J. Org. Chem., cation operations are incorporated
of Captopril using CFS by Chemo- regio-and stereoselective oxidation tion time and excellent yields with a 2017, 6495-6498) have reported the in-line for the Mannich reaction; and In a recent study, Brocklehurst, et
enzymatic Method was demonstrated reaction; and (ii) overall yield of 50% total residence time 90 min for all the formation of fl uconazole using continu- (ii) high production rate of tramadol al. (Org. Process Res. Dev. 2011, 15, 6,
by chlorination step to transform the after crystallisation. four steps. Overall isolated yields for ous fl ow chemistry primarily involving (13.7 g/h). 1447-1453) have reported continuous
chiral intermediate, (R)-3-hydroxy- cinnarizine, cyclizine, and a buclizine the formation of an intermediate via a fl ow nitration of 8-bromo-1H-quinolin-
2-methylpropanoic acid, into the fi nal Use of CFS to undertake a multi- derivative are 82, 94, and 87 %, respec- Grignard reaction, followed by further Melitracen 2-one with near-complete conversion
product. Conducted under continuous step chlorination cascade has been tively). Advantages over batch pro- transformations to obtain fl uconazole. Melitracen is a drug used for the within a 3-minute residence time at
fl ow, this step improves safety and reported with effective inline work- cess include: (i) a four-step continuous treatment of anxiety and depression. 90°C followed by scale-up that yielded
effi ciency by allowing precise control of up and end-of-line crystallisation in process; and (ii) overall good yields. In this protocol, the aryl halide Pederson et al. (Org. Process Res. Dev. 201 g of product. Another continuous
reactive chlorinating agents and in-line batch, leading to isolation of α-thio- reacts with iPrMgCl·LiCl to form the 2018, 22, 2, 228–235) reported a con- nitration protocol was established for
separation of by-products. The seam- β-chloroacrylamide Z-3 in pure form Prieschl et al. (Org Process Res Dev aryl magnesium reagent (a Grignard tinuous fl ow process for synthesis of 2-amino-4-bromo-benzoic acid methyl
less integration of chlorination with from a complex reaction mixture, ex- 2023, 27 (4), 601-609) have reported a reagent) in a continuous fl ow reac- Melitracen. In fl ow process both hydro- ester as the batch nitration results in
subsequent steps makes it almost tele- ploiting the advantage of effi cient heat new route for synthesizing the canna- tor, which subsequently, reacts with 1, lysis and dehydration were performed noticeable quantity of by-product. Further
scopic, thereby eliminating the need for transfer in fl ow. Using a greener solvent binoid receptor type 2 agonist. The fi nal 3-dichloroacetone in the continuous in single step. nitration of 1-benzosuberone in fl ow
synthesis strategy has four steps that fl ow system to form the key intermedi- helped avoid dangerous decomposition
combine batch and fl ow. After batch ate (fl uconazole precursor) with high The phase separation step was also of the accumulated mono-nitro deriva-
N-pivaloylation, high-temperature selectivity and high yield. The inter- eliminated and only THF was used as tive in the presence of excess nitrating
cyclisation was performed in fl ow, mediate is then reacted with 1,2,4-tria- a solvent in comparison to the batch agent and improved the reaction’s
yielding 82% over two steps. In a novel zole in the presence of a base to form process, which requires toluene-THF selectivity.
approach, chlorination using oxalyl fl uconazole. The last step is optimised solvent mixture. The both Grignard
chloride has been demonstrated with in batch conditions due to longer reaction (Step 1), hydrolysis and dehy- The entire approach offered the
a continuous cation exchange workup reaction times. Advantages over batch dration (Step 2) were performed at following advantages over batch protocol:
to prevent side products followed by process include: (i) safety concerns ambient temperature, whereas the batch (i) scaling up nitrations to produce
reaction of the aryl chloride with S-(3)- involved with organometallic reagents, process reaction happens at 50 C. a few hundreds of grams; and (ii) a
o
hydroxypyrrolidine, yielding 88% over addressed through a continuous fl ow safer alternative to running dangerous
two steps. The overall yield for the four approach; and (ii) enhanced window When compared to the batch process, exothermic reactions in short time,
186 Chemical Weekly December 17, 2024 Chemical Weekly December 17, 2024 187
Contents Index to Advertisers Index to Products Advertised