Page 176 - CW E-Magazine (26-11-2024)
P. 176
Special Report Special Report
Bio/Pharma watchlist: Small-molecule drugs
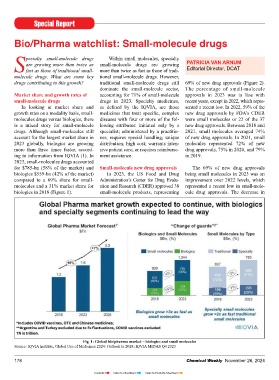
pecialty small-molecule drugs Within small molecules, specialty
are growing more than twice as small-molecule drugs are growing PATRICIA VAN ARNUM
Sfast as those of traditional small- more than twice as fast as those of tradi- Editorial Director, DCAT
molecule drugs. What are some key tional small-molecule drugs. However,
drugs contributing to this growth? traditional small-molecule drugs still 69% of new drug approvals (Figure 2).
dominate the small-molecule sector, The percentage of small-molecule
Market share and growth rates of accounting for 71% of small-molecule approvals in 2023 was in line with
small-molecule drugs drugs in 2023. Specialty medicines, recent years, except in 2022, which repre-
In looking at market share and as defi ned by the IQVIA, are those sented a recent low. In 2022, 59% of the
growth rates on a modality basis, small- medicines that treat specifi c, complex new drug approvals by FDA’s CDER
molecules drugs versus biologics, there diseases with four or more of the fol- were small molecules or 22 of the 37 Fig. 2: Percentage of new drug approvals by the CDER that were small molecules, 2019-2023
is a mixed story for small-molecule lowing attributes: initiated only by a new drug approvals. Between 2018 and Source: CDER
drugs. Although small-molecules still specialist; administered by a practitio- 2021, small molecules averaged 74% small molecules’ share of new drug mechanism of action than existing sclerosis (ALS, i.e., Lou Gehrig’s
account for the largest market share in ner; requires special handling; unique of new drug approvals. In 2021, small approvals in 2022 was largely due to the drugs. disease);
2023 globally, biologics are growing distribution; high cost; warrants inten- molecules represented 72% of new overall decline in new drug approvals 5. GlaxoSmithKline’s Jesduvroq
more than three times faster, accord- sive patient care; or requires reimburse- drug approvals, 75% in 2020, and 79% in 2022 and a corresponding decline In 2023, FDA’s CDER approved 20 (daprodustat) for treating anaemia
ing to information from IQVIA (1). In ment assistance. in 2019. in small-molecule drug approvals and new drugs that it characterized as fi rst- due to chronic kidney disease;
2023, small-molecules drugs accounted a rise in new biologic drug approvals. in-class, which represented approxi- 6. Novartis’ Fabhalta (iptacopan) for
for $785-bn (58% of the market) and Small-molecule new drug approvals The 69% of new drug approvals In 2022, FDA’s CDER approved 22 mately 36% of new drug approvals. Of treating paroxysmal nocturnal haemo-
biologics $559-bn (42% of the market) In 2023, the US Food and Drug being small molecules in 2023 was an new small-molecule drugs and 15 new these 20 fi rst-in-class new drug appro- globinuria, a rare blood disorder;
compared to a 69% share for small- Administration’s Center for Drug Evalu- improvement over 2022 levels, which biologics. The 17 new biologics appro- vals, 17 were small molecules, repre- 7. Novo Nordisk’s Rivfl oza (nedosiran)
molecules and a 31% market share for ation and Research (CDER) approved 38 represented a recent low in small-mole- vals in 2023 surpassed 2022 levels and senting 85% of fi rst-in-class new drug for treating primary hyperoxaluria, a
biologics in 2018 (Figure 1). small-molecule products, representing cule drug approvals. The decrease in matched a recent high in 2018, when approvals in 2023 by FDA’s CDER. Of rare condition characterized by recur-
17 new biologics were also approved these 17 fi rst-in-class, small-molecule rent kidney and bladder stones; and
by FDA’s CDER. The 17 new biologic new drug approvals in 2023, eight were 8. Pfi zer’s Paxlovid (nirmatrelvir tab-
drug approvals in 2023 far exceeded from large to mid-sized bio/pharma lets; ritonavir tablets, co-packaged)
approvals of new therapeutic biologics companies. These eight drugs were: for treating COVID-19.
by FDA’s CDER of 14 in 2021, 13 in 1. Astellas’ Veozah (fezolinetant) for
2020, and 10 in 2019. reducing moderate-to-severe vaso- Although small-molecule drugs
motor symptoms due to menopause; were well represented with 85% of
Product innovation: small-molecules 2. AstraZeneca’s Truqap (capivasertib) the fi rst-in-class new drug approvals
and fi rst-in-class drugs for treating advanced HR-positive in 2023, more than half of these drugs
Aside from just the overall number breast cancer; were for niche indications. Of the 17
of new drug approvals, product innova- 3. Bausch and Lomb’s Miebo (per- small-molecule first-in-class drug
tion can also be evaluated by the num- fl uorohexyloctane ophthalmic solu- approvals in 2023, nine, or 53%, were for
ber of new drug approvals classifi ed as tion) for treating dry-eye disease; treating orphan/rare diseases, defi ned as
“fi rst-in-class,” which FDA’s CDER 4. Biogen’s Qalsody (tofersen) a disease affecting 200,000 individuals
characterizes as drugs with a different for treating amyotrophic lateral or less in the US.
Chemical Weekly | Import-Export Data | Market Surveys
Directories | Business Forums | Expositions
The only organisation in India catering exclusively to the needs of the entire chemical industry
Contact:
SEVAK PUBLICATIONS PVT. LTD.
602-B, Godrej Coliseum, K.J. Somaiya Hospital Road, Behind Everard Nagar, Sion (E), Mumbai 400 022.
Fig. 1: Global bio/pharma market – biologics and small molecules Phone: +91-22-24044471 / 72; Email: admin@chemicalweekly.com
Source: IQVIA Institute, Global Use of Medicines 2024: Outlook to 2028; IQVIA MIDAS Q4 2023
176 Chemical Weekly November 26, 2024 Chemical Weekly November 26, 2024 177
Contents Index to Advertisers Index to Products Advertised