Page 179 - CW E-Magazine (4-3-2025)
P. 179
Special Report Special Report
Recognizing, preventing and treating pitting and fretting steels, general corrosion of the complete
surface is prevented and localized cor-
corrosion rosion at some spots ensues. Whenever
the protective oxide fi lm is minutely
punctured or damaged due to corrosion
INTRODUCTION relatively small. Generally, a pit may be DR. S.K. CHAKRAVORTY or from a mechanical cause and it does
hough barely visible, pitting cor- described as a cavity or hole with the sur- Consultant (Plant Engineering) not heal itself, it develops localized pit-
rosion can have serious implica- face diameter about the same as or less Email: chakravorty4410@gmail.com ting. In this case, the damaged oxide fi lm
Ttions because the cavity it bores than the depth. Figure-1 shows different becomes a cathode and the tiny area of
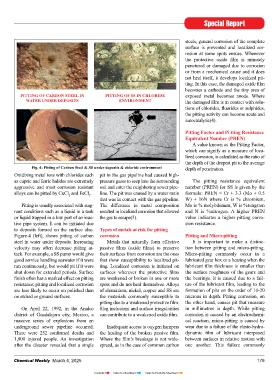
below the surface can be both wide and types of pits formed on the corroding fi cult to measure and predict by labora- PITTING OF CARBON STEEL IN PITTING OF SS IN CHLORIDE exposed metal becomes anode. Where
deep, thus seriously affecting the load surface, such as narrow deep, elliptical, tory tests, hence failures often occur with WATER UNDER DEPOSITS ENVIRONMENT the damaged fi lm is in contact with solu-
carrying capacity of the structure. Pitting wide shallow, subsurface, under cutting, extreme suddenness. As a class, stainless tions of chlorides, fl uorides or sulphides,
corrosion initially presents itself as one or horizontal, and vertical. steel (SS) alloys are more susceptible to the pitting activity can become acute and
more tiny holes or cavities that are nearly damage by pitting corrosion than are any autocatalytic(4).
invisible to the human eye. But this type It is often diffi cult to detect pits be- other group of metals and alloys. Figure-2
of corrosion can quickly progress in both cause of their small size and is often shows pitting of 18-8 SS by sulphuric Pitting Factor and Pi tting Resistance
horizontal and vertical directions below covered with corrosion products. It is dif- acid containing ferric chloride. Equivalent Number (PREN)
the substrate’s surface and can result in A value known as the Pitting Factor,
failures and breakdowns with disastrous A failed 304 type (16% Cr) SS con- which can signify as a measure of loca-
consequences. Some of the deadly bridge denser tube due to pitting corrosion lead- lized corrosion, is calculated as the ratio of
collapses in history are attributed to deep ing to small pinhole leaks is shown in the depth of the deepest pit to the average
or wide cavities because of pitting(1, 2). Figure-3. The tube contained circulating Fig. 4: Pitting of Carbon Steel & SS under deposits & chloride environment depth of penetration.
water for cooling nitric acid in a plant. Oxidizing metal ions with chlorides such pit in the gas pipeline had caused high-
Pitting is a form of extremely loca- The outside of the tube (bottom) was as cupric and ferric halides are extremely pressure gases to seep into the surrounding The pitting resistance equivalent
lized attack that results in holes in the exposed to the process side, or nitric aggressive and most corrosion resistant soil and enter the neighboring sewer pipe- number (PREN) for SS is given by the
metal. These holes may be small or large Fig. 2: Pitting of 18-8 SS by sulphuric acid acid side, and no measurable corrosion alloys can be pitted by CuCl and FeCl . line. The pit was caused by a water main formula: PREN = Cr + 3.3 (Mo + 0.5
2
3
in diameter, but in most cases they are containing ferric chloride occurred on this surface. The cooling that was in contact with the gas pipeline. W) + 16N where Cr is % chromium,
Pitting is usually associated with stag- The difference in metal composition Mo is % molybdenum, W is %tungsten
nant conditions such as a liquid in a tank resulted in localized corrosion that allowed and N is %nitrogen. A higher PREN
or liquid trapped in a low part of an inac- the gas to escape(3). value indicates a higher pitting corro-
tive pipe system. It can be initiated due sion resistance.
to deposits formed on the surface also. Types of metals at risk for pitting
Figure-4 (left), shows pitting of carbon corrosion Pitting and Micro-pitting
steel in water under deposits. Increasing Metals that naturally form effective It is important to make a distinc-
velocity may often decrease pitting at- passive fi lms (oxide fi lms) to preserve tion between pitting and micro-pitting.
Fig. 3: A leaking 304 SS condenser tube due to tack. For example, a SS pump would give their surfaces from corrosion are the ones Micro-pitting commonly occur in a
pitting corrosion
good service handling seawater if it were that show susceptibility to localized pit- lubricated gear box or a bearing when the
water circulating in the tube contained a run continuously, but would pit if it were ting. Localized corrosion is initiated on lubricant fi lm thickness is smaller than
small amount of chlorides. Pitting started shut down for extended periods. Surface surfaces wherever the protective fi lms the surface roughness of the gears and
on the inside (upper) surface and pro- fi nish often has a marked effect on pitting are weakened or broken in one or more the bearings. It is caused due to a fail-
gressed outwards making holes in the resistance; pitting and localized corrosion spots and do not heal themselves. Alloys ure of the lubricant fi lm, leading to the
bottom surface leading to leak. are less likely to occur on polished than of aluminium, nickel, copper and SS are formation of pits on the order of 10-20
on etched or ground surfaces. the materials commonly susceptible to microns in depth. Pitting corrosion, on
From a practical standpoint, most pitting due to a weakened protective fi lm. the other hand, causes pit that measure
pitting failures are caused by chloride On April 22, 1992, in the Analco Slag inclusions and surface irregularities in millimetres in depth. While pitting
and chlorine – containing ions. Chlorides district of Guadalajara city, Mexico, a can contribute to a weakened oxide fi lm. corrosion is caused by an electrochemi-
are present in varying degrees in most massive series of explosions from an cal reaction, micro-pitting is caused by
waters and solutions made with water. Also underground sewer pipeline occurred. Inadequate access to oxygen hampers wear due to a failure of the elasto-hydro-
much equipment operates in seawater and There were 252 confi rmed deaths and the healing of the broken passive fi lm. dynamic fi lm of lubricant interposed
brackish waters. Figure-4 (right), shows 1,800 injured people. An investigation Where the fi lm’s breakage is not wide- between surfaces in relative motion with
Fig. 1: Types of pits formed in pitting corrosion pitting of SS in chloride environment. after the disaster revealed that a single spread, as in the case of common carbon one another. This failure commonly
178 Chemical Weekly March 4, 2025 Chemical Weekly March 4, 2025 179
Contents Index to Advertisers Index to Products Advertised