Page 171 - CW E-Magazine (31-12-2024)
P. 171
Special Report Special Report
A shortage of inspectors curtailing FDA inspections: Inspections address investigator vacancies; partial
fi scal year 2024 was the most recently
The impact on pharma 2,000 COVID-19 public health emergency, available data when GAO conducted
its analysis. GAO also interviewed
January 2020 – May 2023
July 2021 normal domestic offi cials about their efforts to maintain a
DA conducted 621 foreign and inspection responsibilities were al- 1,500 inspection operations resume suffi cient pool of drug investigators,and
444 domestic inspections in fi s- ready complicated by a manufacturing PATRICIA VAN ARNUM March 2022 normal it reviewed FDA documents related
foreign inspection
Fcal year 2023, which was up supply chain that has become increa- Editorial Director, DCAT operations resume to workforce planning and investiga-
from pandemic levels when in-person singly global. As of September 2023, 1,000 tor recruitment and hiring. In addition,
inspections were paused, but 36% fewer 58% of establishments registered pandemic, but inspection totals remained GA interviewed investigators regarding
than pre-pandemic levels. A shortage of with FDA to manufacture drugs for below pre-pandemic totals due to re- 500 their experiences conducting in-person
inspectors is a major factor. What’s the the US market were located overseas, duced investigator capacity. As a part of inspections, including challenges
impact on Pharma?. according to a recent analysis by the resuming in-person inspections, FDA related to inspection staffi ng. GAO
US Government Accountability Offi ce implemented several pilot measures 0 compared FDA’s efforts and action
A two-fold problem (GAO) (GAO is an independent, non- to facilitate the inspection of foreign 2016 2017 2018 2019 2020 2021 2022 2023 plans to maintain its drug investigator
The COVID-19 pandemic signi- partisan government agency within drug establishments, including a pilot Fiscal year workforce against selected leading
fi cantly disrupted the ability of the the legislative branch that provides program for unannounced inspections Domestic practices for retention previously iden-
US Food and Drug Administration’s auditing, evaluative, and investigative of foreign facilities and fi nalized the Foreign tifi ed by GAO.
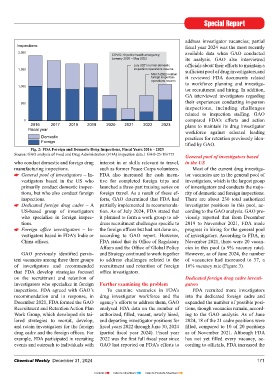
(FDA) to inspect drug manufacturing services for the US Congress). These design of the independent interpreter Fig. 2: FDA Foreign and Domestic Drug Inspections, Fiscal Years 2016 – 2023
establishments in person. Beginning include establishments making both pilot. Source: GAO analysis of Food and Drug Administration (FDA) inspection data. / GAO-25-106775 General pool of investigators based
in March 2020, FDA largely paused active pharmaceutical ingredients and who conduct domestic and foreign drug interest in or skills relevant to travel, in the US
foreign and domestic drug inspections. fi nished dosage forms. As of January In fi scal year 2023, FDA conducted manufacturing inspections. such as former Peace Corps volunteers. Most of the current drug investiga-
In response to these disruptions, FDA 2024, FDA data showed that India and more drug inspections than it had since General pool of investigators – In- FDA also increased the cash incen- tor vacancies are in the general pool of
relied on the use of alternative inspection China had the most foreign establish- pausing most inspections in March vestigators based in the US who tive for completed foreign trips and investigators, which is the largest group
tools. Such tools included the review ments manufacturing drugs for the US 2020, at the start of the COVID- 19 pan- primarily conduct domestic inspec- launched a three-part training series on of investigators and conducts the majo-
of inspection reports from other trusted market, with nearly 40% of all foreign demic, but did not reach pre-pandemic tions, but who also conduct foreign foreign travel. As a result of these ef- rity of domestic and foreign inspections.
foreign regulators and the remote establishments in these two countries inspection totals. These data show FDA inspections. forts, GAO determined that FDA had There are about 230 total authorized
assessment of information from manu- (Figure 1). conducted 1,065 total inspections in Dedicated foreign drug cadre – A partially implemented its recommenda- investigator positions in this pool, ac-
facturing establishments. While FDA fi scal year 2023, a 40% increase from US-based group of investigators tion. As of July 2024, FDA stated that cording to the GAO analysis. GAO pre-
generally had the authority to use these Inspections of domestic versus fi scal year 2022. However, this total who specialize in foreign inspec- it planned to form a work group to ad- viously reported that from December
tools prior to the pandemic, it had not foreign manufacturing facilities was 36% below the 1,671 inspections tions. dress recruitment challenges specifi c to 2019 to November 2021, FDA made
used them extensively. By the start of fi scal year 2023, conducted in fi scal year 2019, the last Foreign offi ce investigators – In- the foreign offi ces but had not done so., progress in hiring for the general pool
FDA had resumed conducting in- full year before the start of the pandemic vestigators based in FDA’s India or according to GAO report. However, of investigators. According to FDA, in
Prior to the pandemic, however, person inspections paused during the (Figure 2). China offi ces. FDA stated that its Offi ce of Regulatory November 2021, there were 20 vacan-
Affairs and the Offi ce of Global Policy cies in this pool (a 9% vacancy rate).
The number of inspections FDA GAO previously identifi ed persis- and Strategy continued to work together However, as of June 2024, the number
United conducted was limited by reduced tent vacancies among these three groups to address challenges related to the of vacancies had increased to 37, a
Canada Kingdom investigator capacity, according to FDA
France Germany of investigators and recommended recruitment and retention of foreign 16% vacancy rate (Figure 3).
United States Spain Italy offi cials. In its report, GAO said that that FDA develop strategies focused offi ce investigators.
China Japan
FDA offi cials told the GAO that they on the recruitment and retention of Dedicated foreign drug cadre investi-
India South Korea anticipate that fi scal year 2024 inspec- investigators who specialize in foreign Further examining the problem gators
tion totals will be higher than fi scal year inspections. FDA agreed with GAO’s To examine vacancies in FDA’s FDA recruited more investigators
2023 but will remain below pre-pan- recommendation and in response, in drug investigator workforce and the into the dedicated foreign cadre and
demic levels due to continued reduced December 2021, FDA formed the GAO agency’s efforts to address them, GAO expanded the number of possible posi-
investigator capacity. Recruitment and Retention Action Plan analysed FDA data on the number of tions, though vacancies remain, accord-
Work Group, which developed six tai- authorized, fi lled, vacant, newly hired, ing to the GAO analysis. As of June
FDA’s inspection workforce lored strategies to recruit, develop, and departing investigator positions for 2024, 18 of the 21 cadre positions were
Manufacturing Establishments 1500+ 400-600 100-399 79-99 To understand this inspector short- and retain investigators for the foreign fi scal years 2022 through June 30, 2024 fi lled, compared to 10 of 20 positions
Fig.1: Number of Establishments in the US and the 10 Countries with the Most Foreign Drug age, it is fi rst important to know how drug cadre and the foreign offi ces. For (partial fi scal year 2024). Fiscal year as of November 2021. Although FDA
Establishments Manufactruring Drugs for the US Market, as of January 2024. FDA staffs its inspection workforce. example, FDA participated in recruiting 2022 was the fi rst full fi scal year since has not yet fi lled every vacancy, ac-
Source: GAO analysis of Food and Drug Administration data (data); National Atlas (base map). /GAO-25-106775 FDA has three groups of investigators events and outreach to individuals with GAO last reported on FDA’s efforts to cording to offi cials, FDA increased the
170 Chemical Weekly December 31, 2024 Chemical Weekly December 31, 2024 171
Contents Index to Advertisers Index to Products Advertised