Page 181 - CW E-Magazine (1-4-2025)
P. 181
Special Report
Hydrogen damage in materials
IntroductIon Dr. S.K. ChaKravorty
he presence of atomic hydrogen Consultant (Plant Engineering)
in a medium is extremely dan- Email: chakravorty4410@gmail.com
Tgerous and can cause serious
damage to materials. The term “hydro- Mechanism of hydrogen damage
gen damage” refers to the mechanical Hydrogen causes premature crack-
damage of metal caused by interac- ing and lowers ductility and toughness
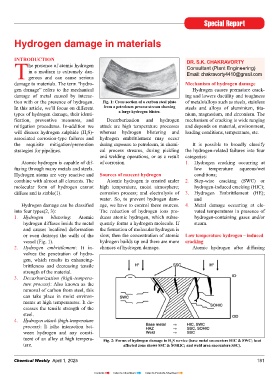
tion with or the presence of hydrogen. Fig. 1: cross section of a carbon steel plate of metals/alloys such as steels, stainless
In this article, we’ll focus on different from a petroleum process stream showing steels and alloys of aluminium, tita-
types of hydrogen damage, their identi- a large hydrogen blister. nium, magnesium, and zirconium. The
fication, preventive measures, and Decarburization and hydrogen mechanism of cracking is wide ranging
mitigation procedures. In-addition we attack are high temperature processes and depends on material, environment,
will discuss hydrogen sulphide (H S)- whereas hydrogen blistering and loading conditions, temperature, etc.
2
associated corrosion-type failures and hydrogen embrittlement may occur
the requisite mitigation/prevention during exposure to petroleum, in chemi- It is possible to broadly classify
strategies for pipelines. cal process streams, during pickling the hydrogen-related failures into four
and welding operations, or as a result categories:
Atomic hydrogen is capable of dif- of corrosion. 1. Hydrogen cracking occurring at
fusing through many metals and steels. low temperature aqueous/wet
Hydrogen atoms are very reactive and Sources of nascent hydrogen conditions;
combine with almost all elements. The Atomic hydrogen is created under 2. Step-wise cracking (SWC) or
molecular form of hydrogen cannot high temperature, moist atmosphere; hydrogen-induced cracking (HIC);
diffuse and is stable(1). corrosion process; and electrolysis of 3. Hydrogen Embrittlement (HE);
water. So, to prevent hydrogen dam- and
Hydrogen damage can be classified age, we have to control these sources. 4. Metal damage occurring at ele-
into four types(2, 3): The reduction of hydrogen ions pro- vated temperatures in presence of
1. Hydrogen blistering: Atomic duces atomic hydrogen, which subse- hydrogen-containing gases and/or
hydrogen diffuses inside the metal quently forms a hydrogen molecule. If steam.
and causes localized deformation the formation of molecular hydrogen is
or even destroys the walls of the slow, then the concentration of atomic Low temperature hydrogen – induced
vessel (Fig. 1). hydrogen builds up and there are more cracking
2. Hydrogen embrittlement: It in- chances of hydrogen damage. Atomic hydrogen after diffusing
volves the penetration of hydro-
gen, which results in enhancing-
brittleness and decreasing tensile
strength of the material.
3. Decarburization (high-tempera-
ture process): Also known as the
removal of carbon from steel, this
can take place in moist environ-
ments at high temperatures. It de-
creases the tensile strength of the
steel.
4. Hydrogen attack (high-temperature
process): It isthe interaction bet-
ween hydrogen and any consti-
tuent of an alloy at high tempera- Fig. 2: Forms of hydrogen damage in H S service (base metal encounters HIc & SWc; heat
2
ture. affected zone shows SSc & SoHIc; and weld area encounters SSc).
Chemical Weekly April 1, 2025 181
Contents Index to Advertisers Index to Products Advertised