Page 181 - CW E-Magazine (14-5-2024)
P. 181
Special Report Special Report
4-hydroxymandelic acid to 4-hydroxy- area of genome-mining and prepara- logical science. An example of beta- for conversion of dehydromahonial
phenylglyoxylic acid (4-HPGA) with tion of novel recombinant enzymes for hydroxy-alpha amino acid chiral build- to Mahonial. Two phase systems have
enhanced activity. (S)-Enantioselective synthesis of lead molecules, drug candi- ing blocks is covered. also been reported.
HMO mutant A80G-T159S-T162Q dates, and APIs. Enzyme engineering
(HMOTM) was used together with activities were undertaken. Enzymes There is a considerable coverage on Alcohol dehydrogenases (ADHs) are
mandelic acid racemase (MR) and cata- are now used for not only for making enzymes in the manufacture of APIs. discussed, e.g., using alcohol oxidases.
lase (KATE) to realise high-yielding chiral molecules, but many molecules Examples include Cipargamin (a very Fatty aldehydes from fatty alcohols
oxidation of racemic-4-hydroxyman- in a facile way. These authors have effective anti-malaria drug) where one have been reported. Racemic resolution
delic acid to 4-HPGA. Other substituted covered how the science has evolved key intermediate is a chiral tryptamine of undecavertol using an (S)-selective
MAs were also studied. Thus, effi - over the last four decades. where a transaminase is used; Sacu- ADH is reported.
cient conversion of racemic-α-hydroxy There is a coverage on the early ap- bitril (the active ingredient of Entresto). Recent advances in computational
There are examples of multi-tonne pro-
acids to (S)-or (R)-α-amino acids were proaches to chiral building blocks. ducts. Examples of enantioselectivity enzyme engineering are very encour-
realised. (ACS Catal., 2023, 13, 1522- Hydrolytic resolutions received attention reversal are given. Chemo-enzymatic aging. Artifi cial intelligence will not
1532; DOI: 10.1021/acscatal.2c05596).
and chiral non-natural amino acids, such synthesis of EMA 401 is covered; the completely replace the need for experi-
Evolution of biocatalysis at as D-tert-leucine, phenylalanine deriva- key intermediate involved asymmetric ments, but will signifi cantly reduce
their numbers.
tives, etc., were developed. Kinetic reso-
Novartis over the last 40 years lutions of chiral amino acids synthesis hydrogenation.
are covered. Asymmetric reductions Development of enzymatic solutions Development of an amine
E. Siirola et al have given a fascina- and in 1991, (R)-2-hydroxyl-4-phenyl- for increased molecular complexity is transaminase-lipase cascade
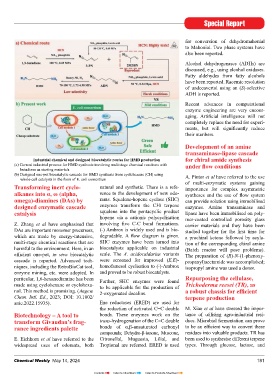
tion account of the development in the butyric acid via the reduction of the cor- covered. (Chimia, 2023, 77, No.6, 376- Industrial chemical and designed biocatalytic routes for HMD production for chiral amide synthesis
world-renowned Novartis and anyone responding keto acid, was developed. 383; DOI:10.2533/chimia.2023.376). (a) Current industrial process for HMD synthesis involving multistage chemical reactions with under fl ow conditions
interested in this subject should read Engineered keto reductase were used in butadiene as starting materials
this paper. The microbial strains built pursuit of directed evolution. Metabolite Enantioselective switch and (b) Designed one-pot biocatalytic cascade for HMD synthesis from cyclohexane (CH) using A. Pintor et al have referred to the use
during the 1980’s for degradation of synthesis has received attention, e.g., of potential applications in bio- whole-cell catalysts in the form of E. coli consortium of multi-enzymatic systems gaining
DMF, dimethyl sulphate, etc., helped Siponimod and Leniolisib. catalysis Transforming inert cyclo- natural and synthetic. There is a refe- importance for complex asymmetric
the company to switch from biodegra- alkanes into α, ω (alpha, rence to the development of new odo- syntheses and the use of fl ow system
dation to the use of enzymes for syn- Late stage functionalisation of clados- L. Robustini and F. Paradisi have omega)-diamines (DAs) by rants. Squalene-hopene cyclase (SHC) can provide solution using immobilised
thetic purposes. The discovery of novel porin is reported. There is a coverage emphasised the importance of enzymes, designed enzymatic cascade enzymes transform the C30 terpene enzymes. Amine transaminase and
enzymes internally and also through of a new era of Enzyme Discovery and which being chiral in nature are highly catalysis squalene into the pentacyclic product lipase have been immobilised on poly-
external companies and collaboration Engineering. This refers to the impact selective when applied to their natu- hopene via a cationic polycyclisation mer-coated controlled porosity glass
partners gave useful tools. Molecular of genome sequencing coupled with ral substrates and the selectivity tends Z. Zhang et al have emphasised that involving fi ve C-C bond formations. carrier materials and they have been
biology, DNA-reading and writing, recent advances in DNA synthesis tech- DAs are important monomer precursors, (-) Ambrox is widely used and is bio- studied together for the fi rst time for
and the development of bioinformatics nologies and has had a revolutionary which are made by energy-intensive, degradable. A fl ow diagram is given. a prochiral ketone followed by acyla-
tools allowed to further expand into the impact on several disciplines of bio- multi-stage chemical reactions that are SHC enzymes have been turned into tion of the corresponding chiral amine
harmful to the environment. Here, in an biocatalysts applicable on industrial (Batch reactor will pose problems).
effi cient one-pot, in vivo biocatalytic scale. The A. acidocaldarius variants The preparation of (R)-N-(1-phenoxy-
ZPL389 cascade is reported. Advanced tech- were screened for improved (E,E)- propanyl)acetamide was accomplished;
2021 to remain when non-natural substrates
LNP023 niques, including the RetroBioCat tool, homofarnesol cyclisation to (-)-Ambrox
2019 isopropyl amine was used a donor.
AFQ056 are used. Examples are given to show enzyme mining, etc. were adopted. In and proved to be robust biocatalysts.
2017
KAE609 2015 differences between enantiomers. Thus particular, 1,6-hexanediamine has been Repurposing the cellulase,
2010 S-(+)-carvone is mentholated, to give Further, SHC enzymes were found
1982 1999 2005 made using cyclohexane or cyclohexa- to be applicable for the production of Trichoderma reesei (TR), as
a spicy aroma with bready notes and nol. This method is promising. (Angew. a robust chassis for effi cient
medium strength; R-(-)-carvone has Chem. Intl. Ed., 2023; DOI: 10.1002/ 2-oxygenated decalins.
ENA713 PIM477 terpene production
EMA401 herbal, minty and sweetish medium anie.2022.15935). Ene reductases (ERED) are used for
CQC959
SPP100 strength fragrance. A variety of trans- the reduction of activated C=C double M. Xiao et al have stressed the impor-
Hydrolases aminase allows, e.g., CH COCH CH bonds. These enzymes work on the tance of utilising agro-industrial resi-
Transaminases 3 2 3 Biotechnology – A tool to
Ketoreductase to the corresponding chiral amine; THF transform Givaudan’s frag- trans-hydrogenation of the C=C double dues. Microbial fermentation can prove
Ene reductase
Phenylalanine ketone to THF amine. Different methods bonds of α,β-unsaturated carbonyl to be an effi cient way to convert these
ammonia lyase Imine rance ingredients palette
reductase of enantioselective switch are discussed. compounds; Dehydro-β-ionone, Muscone, residues into valuable products. TR has
The fi rst tailored enzyme by protein engineering was used for the synthesis of intermediate of (Chimia, 2023, 77, No.6, 390-394: E. Eichhorn et al have referred to the Citronellal, Muguesia, Lilial, and been used to synthesise different terpene
KAE609 in 2010 DOI:10.2533/chimia.2023.390). widespread uses of odorants, both Tropional are referred. ERED is used types. Through glucose, lactose, and
180 Chemical Weekly May 14, 2024 Chemical Weekly May 14, 2024 181
Contents Index to Advertisers Index to Products Advertised