Page 141 - CW E-Magazine (8-7-2025)
P. 141
Pharmaceuticals Pharmaceuticals
CONSOLIDATION INORGANIC GROWTH
Torrent Pharma to acquire controlling stake in JB IMCD bolsters pharma portfolio with Trichem
Chemicals & Pharmaceuticals from KKR acquisition
Torrent Pharmaceuticals is acquir- cial release. Torrent has also expressed Chairman, Torrent Pharmaceuticals, stated, Dutch chemicals and ingredients
ing a controlling stake in JB Chemi- its intent to acquire up to 2.80 percent “Torrent’s deep India presence and distributor, IMCD, has announced the
cals & Pharmaceuticals from New of equity shares from certain emplo- JB Pharma’s fast growing India busi- acquisition of 100% of the shares in
York-based private equity fi rm, yees of JB Pharma at the same price ness, combined with the CDMO and Trichem Healthcare Pvt. Ltd., Trichem
KKR, for a purchase price of up to per share as KKR. international footprint off ers immense Lifesciences Ltd. and Chemistry &
Rs. 19,480-crore, implying an equity potential to scale both revenue and Health FZ LLC.
valuation of Rs. 25,689-crore. The open off er price is higher than profi tability. This strategic alignment
the purchase price for KKR and the furthers our goal of strengthening our Founded in 1998 and headquartered
The acquisition will be followed by employees of JB Pharmaceuticals. On presence in the Indian pharma market, in Mumbai, Trichem supplies active
a merger of JB Chemicals with Torrent the BSE, JB Chemicals and Pharma- and build a larger diversifi ed global pharmaceutical ingredients, pharma-
Pharmaceuticals, a joint statement said. ceuticals had closed at a price of presence. Moreover, the CDMO plat- ceutical intermediates, and formulation
Rs. 1,799.35 per share on June 27. form provides a new long-term avenue solutions, besides providing regulatory
The Ahmedabad-based fl agship KKR has made fi ve times return on of growth for Torrent.” assistance for customers serving regu-
company of the Torrent Group stated its investment in JB Pharmaceuticals lated pharmaceutical markets in India.
that the transaction will be executed with an internal rate of return of Mr. Nikhil Chopra, CEO and Whole
in two phases. The acquisition of 36 per cent. Time Director of JB Pharma, said, With 36 employees, Trichem ope-
46.39 percent equity stake (on a fully “Over the past fi ve years, JB Pharma rates across India and the Middle East,
diluted basis) through a Share Pur- As per the approval given by the has emerged as one of India’s fastest generating annual revenue of approxi-
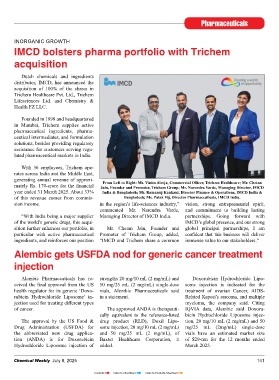
From Left to Right: Ms. Vinita Alreja, Commercial Offi cer, Trichem Healthcare; Mr. Chetan
chase Agreement at a consideration of board of directors of both companies, growing pharmaceutical players. As we mately Rs. 170-crore for the fi nancial Jain, Founder and Promoter, Trichem Group; Mr. Narendra Varde, Managing Director, IMCD
Rs. 11,917-crore (Rs. 1,600 per share) upon merger of JB Pharma with Torrent now enter a new chapter alongside Tor- year ended 31 March 2025. About 33% India & Bangladesh; Mr. Ramanuj Kankani, Director Finance & Operations, IMCD India &
followed by a mandatory open off er to Pharmaceuticals, every shareholder rent Pharmaceuticals, we are confi dent of this revenue comes from commis- Bangladesh; Ms. Palak Vij, Director Pharmaceuticals, IMCD India.
acquire up to 26 percent of JB Pharma holding 100 shares in JB Pharma shall that the combined strengths of our sion income. in the region’s life-sciences industry,” vision, strong entrepreneurial spirit,
shares from public shareholders at an receive 51 shares of Torrent. organisations will unlock greater oppor- commented Mr. Narendra Varde, and commitment to building lasting
open off er price of Rs. 1,639.18 per tunities to enhance healthcare access “With India being a major supplier Managing Director of IMCD India. partnerships. Going forward with
share, the company informed in an offi - Mr. Samir Mehta, Executive across our markets”. of the world’s generic drugs, this acqui- IMCD’s global presence, and our strong
sition further enhances our portfolio, in Mr. Chetan Jain, Founder and global principal partnerships, I am
Aurobindo arm resumes operations at fi re hit particular with active pharmaceutical Promoter of Trichem Group, added, confi dent that this business will deliver
ingredients, and reinforces our position “IMCD and Trichem share a common immense value to our stakeholders.”
penicillin-G unit
Alembic gets USFDA nod for generic cancer treatment
Lyfi us Pharma Pvt. Ltd., a wholly- coal crusher area of the Pen-G faci- estimated period for 20 to 25 days,” it
owned step-down subsidiary of lity. “The fi re incident took place due had said. injection
Aurobindo Pharma, has resumed pro- to self-ignition of coal,” the company
duction of Penicillin-G at its manu- had said. The 15,000 metric tonnes per Alembic Pharmaceuticals has re- strengths 20 mg/10 mL (2 mg/mL) and Doxorubicin Hydrochloride Lipo-
facturing facility in Kakinada, Andhra annum capacity plant was set up at an ceived the fi nal approval from the US 50 mg/25 mL (2 mg/mL) single-dose some injection is indicated for the
Pradesh. This follows the receipt of “The incident resulted in damage investment of Rs 2,500-crore under health regulator for its generic ‘Doxo- vials, Alembic Pharmaceuticals said treatment of ovarian Cancer, AIDS-
the ‘Consent to Operate’ (CTO) from to certain ancillary equipment, with the govt’s production linked incentive rubicin Hydrochloride Liposome’ in- in a statement. Related Kaposi’s sarcoma, and multiple
the Andhra Pradesh Pollution Con- no impact to the core manufacturing (PLI) scheme for key starting materials, jection used for treating diff erent types myeloma, the company said. Citing
trol Board (APPCB) on June 29, infrastructure. Importantly there were no drug intermediates and active pharma- of cancer. The approved ANDA is therapeuti- IQVIA data, Alembic said Doxoru-
2025. injuries reported,” Aurobindo Pharma ceutical ingredients (APIs). The plant, cally equivalent to the reference-listed bicin Hydrochloride Liposome injec-
had said in a regulatory fi ling after the which makes Pen-G for the domestic The approval by the US Food & drug product (RLD), Doxil Lipo- tion, 20 mg/10 mL (2 mg/mL) and 50
The company had temporarily incident. “As a temporary measure and exports market, became operational Drug Administration (USFDA) for some Injection, 20 mg/10 mL (2 mg/mL) mg/25 mL (2mg/mL) single-dose
paused production after a fi re broke and to facilitate necessary equipment in April 2024 but was formally inaugu- the abbreviated new drug applica- and 50 mg/25 mL (2 mg/mL), of vials have an estimated market size
out at the facility on April 27, 2025. replacements, operations at the plant rated by Prime Minister Narendra Modi tion (ANDA) is for Doxorubicin Baxter Healthcare Corporation, it of $29-mn for the 12 months ended
The fi re occurred in the vicinity of the will be temporarily paused for an virtually in October 2024 end. Hydrochloride Liposome injection of added. March 2025.
140 Chemical Weekly July 8, 2025 Chemical Weekly July 8, 2025 141
Contents Index to Advertisers Index to Products Advertised